What Determines the Properties of Each Family on the Periodic Table
The periodic tabular array of the chemical elements is a tabular brandish of the chemical elements. Information technology is perhaps the icon of Chemistry and expresses much about the concrete and chemical properties of the known elements. The emergence of the periodic table occurred concurrently with the development of the scientific understanding of the composition of matter. In its current form, it owes much to quantum mechanics. The electronic structures derived from breakthrough mechanics are used to explicate theoretically the experimentally observed periodic variations in properties of the elements. The periodic table is 1 of the essential components of our understanding of the universe and underlies all of chemistry.
Contents
- 1 History
- 2 Methods for displaying the periodic table
- ii.one Standard periodic table
- ii.ii Other depictions
- three Construction of the Table
- 3.1 Groups
- 3.2 Periods
- 3.3 Electronic structure
- four Come across also
- v References
- 6 External links
- 7 Credits
History
- Main commodity: History of the periodic table
The original table was created without a knowledge of the inner construction of atoms, simply rather by correlating physical and chemical properties of the elements with atomic mass. If the elements are ordered by diminutive mass and so a certain periodicity, or regular repetition, of physical and chemical properties tin be observed. The offset to recognize these regularities was the High german pharmacist Johann Wolfgang Döbereiner who, in 1829, noticed a number of triads of similar elements:
| Element | Molar mass (g/mol) | Density (g/cm³) | Quotient (cm³/mol) |
|---|---|---|---|
| chlorine | 35.4527 | 0.003214 | 11030 |
| bromine | 79.904 | 3.122 | 25.half dozen |
| iodine | 126.90447 | iv.93 | 25.7 |
| calcium | twoscore.078 | 1.54 | 26.0 |
| strontium | 87.62 | two.64 | 33.2 |
| barium | 137.327 | 3.594 | 38.two |
This was followed by the English chemist John Newlands, who noticed in 1865 that the elements of like type recurred at intervals of eight, which he likened to the octaves of music, though his law of octaves was ridiculed by his contemporaries. Finally, in 1869, the German language Julius Lothar Meyer and the Russian chemistry professor Dmitri Ivanovich Mendeleev almost simultaneously developed the kickoff periodic table, arranging the elements by mass. Notwithstanding, Mendeleev plotted a few elements out of strict mass sequence in society to brand a meliorate match to the backdrop of their neighbors in the table. He also corrected mistakes in the values of several atomic masses, and predicted the beingness and backdrop of a few new elements in the empty cells of his tabular array. Mendeleev was subsequently vindicated by the discovery of the electronic structure of the elements in the late nineteenth century and early twentieth century. The modernistic table is based on this understanding of the electronic structures.
In 1913, Henry Moseley rearranged the tabular array co-ordinate to atomic number to improve the observed periodicity in the chemical properties across the table. Today's table uses this ordering by atomic number (number of protons). Mendeleev's and Moseley's development of the periodic table was one of the greatest achievements in modern chemistry. Chemists were able to qualitatively explain the behavior of the elements, and to predict the existence of yet undiscovered ones.
In the 1940s Glenn T. Seaborg identified the transuranic lanthanides and the actinides, which may exist placed within the table, or below (come across the unlike possible arrangements below).
Methods for displaying the periodic tabular array
Standard periodic table
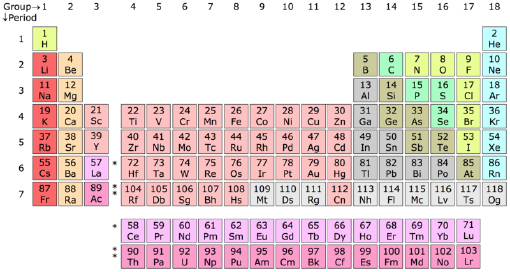
| Group → | i | 2 | 3 | 4 | v | 6 | seven | viii | 9 | 10 | xi | 12 | 13 | 14 | fifteen | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Menstruum ↓ | | |||||||||||||||||||
| i | 1 H | | 2 He | |||||||||||||||||
| 2 | 3 Li | 4 Exist | | 5 B | 6 C | 7 N | eight O | 9 F | 10 Ne | |||||||||||
| three | 11 Na | 12 Mg | | 13 Al | fourteen Si | 15 P | 16 S | 17 Cl | eighteen Ar | |||||||||||
| four | xix K | 20 Ca | 21 Sc | 22 Ti | 23 Five | 24 Cr | 25 Mn | 26 Fe | 27 Co | 28 Ni | 29 Cu | xxx Zn | 31 Ga | 32 Ge | 33 Every bit | 34 Se | 35 Br | 36 Kr | ||
| v | 37 Rb | 38 Sr | 39 Y | xl Zr | 41 Nb | 42 Mo | 43 Tc | 44 Ru | 45 Rh | 46 Pd | 47 Ag | 48 Cd | 49 In | l Sn | 51 Sb | 52 Te | 53 I | 54 Xe | ||
| 6 | 55 Cs | 56 Ba | * | 72 Hf | 73 Ta | 74 W | 75 Re | 76 Os | 77 Ir | 78 Pt | 79 Au | 80 Hg | 81 Tl | 82 Pb | 83 Bi | 84 Po | 85 At | 86 Rn | ||
| 7 | 87 Fr | 88 Ra | ** | 104 Rf | 105 Db | 106 Sg | 107 Bh | 108 Hs | 109 Mt | 110 Ds | 111 Rg | 112 Uub | 113 Uut | 114 Uuq | 115 Uup | 116 Uuh | 117 Uus | 118 Uuo | ||
| | ||||||||||||||||||||
| * Lanthanides | 57 La | 58 Ce | 59 Pr | 60 Nd | 61 Pm | 62 Sm | 63 Eu | 64 Gd | 65 Tb | 66 Dy | 67 Ho | 68 Er | 69 Tm | seventy Yb | 71 Lu | |||||
| ** Actinides | 89 Ac | 90 Th | 91 Pa | 92 U | 93 Np | 94 Pu | 95 Am | 96 Cm | 97 Bk | 98 Cf | 99 Es | 100 Fm | 101 Physician | 102 No | 103 Lr | |||||
| Alkali metals | Element of group i globe metals | Lanthanides | Actinides | Transition metals |
| Poor metals | Metalloids | Nonmetals | Halogens | Noble gases |
Land at standard temperature and force per unit area
- Elements numbered in crimson are gases.
- Elements numbered in green are liquids.
- Elements numbered in black are solids.
Natural occurrence
-
Elements without borders take not been discovered/synthesized yet.
-
Elements with dotted borders do not occur naturally (synthetic elements).
-
Elements with dashed borders naturally arise from decay of other chemic elements.
-
Elements with solid borders are older than the Earth (primordial elements).
- Notation: Although californium (Cf, 98) is not Earth-primordial, it (and its disuse products) does occur naturally: its electromagnetic emissions are regularly observed in supernova spectra.
Other depictions
- The standard table (shown above) provides the basics.
- A vertical tabular array for improved readability in spider web browsers.
- The large table provides the basics plus full chemical element names and atomic masses.
- A tabular array with an inline F-block inserts the lanthanides and actinides back into the table.
- Electron configurations
- Metals and not-metals
- Periodic table filled past blocks
- List of elements past name with atomic number and diminutive mass
- List of elements by electronegativity
- Mendeleev's periodic table
Structure of the Table
Each element appears in a box which contains the symbol of the element and its atomic number. Many tables also include the diminutive mass, and some accept additional information as well. The fundamental ordering of the elements is as a list co-ordinate to their atomic number (number of protons). As of 2005, the table contains 116 chemical elements whose discoveries have been confirmed. Of those 94 are found naturally on Globe, and the balance are synthetic elements that have been produced artificially in laboratories. Following this bones social club the elements are bundled in a table that contains specific columns and rows, known as groups and periods respectively (see the in a higher place table).
Groups
The columns of the table are known as groups or families. All the elements in a group have similar properties. Placing elements in groups is 1 of the most of import ways of classifying them. There is some variation in properties within a group, simply the changes are relatively modest as ane goes down (or up) the grouping. Each group of elements forms what is chosen a chemical series.
In that location are three ways of numbering the groups of the periodic table. The standard International Spousal relationship of Pure and Practical Chemistry (IUPAC) system is to simply number them 1 though eighteen as in the tabular array above. There are also 2 older systems using Roman numerals. The Roman numeral names are the original traditional names of the groups; the standard IUPAC system replaces the quondam names in an effort to reduce the confusion generated by the two older, but mutually disruptive, schemes. Some of the groups have special names (see below). Groups one, 2, thirteen, xiv, xv, 16, 17, and 18 are likewise collectively known every bit the chief group, or representative, elements, and groups 3 through 12 are the transition metals.
There is considerable defoliation surrounding the two old systems in use (old IUPAC and CAS) that combined the use of Roman numerals with letters. In the former IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS system the messages A and B were designated to main group elements (A) and transition metals (B). The sometime system was often used in Europe while the latter was most common in America. The new IUPAC scheme was developed to supercede both systems as they confusingly used the same names to hateful different things.
The periodic table groups are every bit follows (in the brackets are shown the old systems: European and American):
- Group ane (IA,IA): the alkali metals
- Grouping 2 (IIA,IIA): the element of group i earth metals
- Grouping 3 (IIIA,IIIB)
- Group iv (IVA,IVB)
- Grouping 5 (VA,VB)
- Group 6 (VIA,VIB)
- Group vii (VIIA,VIIB)
- Group viii (8)
- Group 9 (Viii)
- Grouping 10 (Viii)
- Group 11 (IB,IB): the coinage metals (not a IUPAC-recommended proper name)
- Grouping 12 (IIB,IIB)
- Group 13 (IIIB,IIIA): the boron group
- Group fourteen (IVB,IVA): the carbon grouping
- Grouping 15 (VB,VA): the pnictogens (not a IUPAC-recommended name) or nitrogen group
- Group sixteen (VIB,VIA): the chalcogens
- Group 17 (VIIB,VIIA): the halogens
- Grouping eighteen (Group 0): the noble gases
Periods
The rows of the tabular array are known every bit periods. It is in the succesive periods that we observe the periodicity of backdrop of the elements. Each period has the full range of properties. For case more metallic elements occur to the left of a period, and the less metallic elements to the right; or oxides of the elements to the left are basic and acidic for elements to the right. The periods are simply numbered ane though vii from the top downwardly
Electronic structure
The shape of the periodic table and the placement of an element in a particular group or period is derived from the electronic structure of the atoms of the element. In fact the chemical and physical properties of an element derive from information technology's electronic construction. Thus it is the electronic structures of the elements that are the source of the observed periodicity of backdrop and the groups and periods of the periodic table.
The electronic structures of the elements derive from quantum mechanics. The quantum mechanical clarification of an atom suggests that the electrons take a circuitous, but precise organization surrounding the diminutive nucleus. The electrons are organized primarily into shells of increasing size and energy, which are numbered sequentially beginning with 1 as the everyman energy. The shells incorporate subshells which tin be represented by letters. The most common subshells are s, p, and d. The subshells are in turn comprised of orbitals, where each orbital can comprise 2 electrons.
Of item importance are the electrons in the highest energy (outermost) shell. These are the electrons that determine the position of the element in the table and are primarily responsible for the properties of the element. In the main grouping elements these outermost electrons are known every bit the valence electrons. The elements in a given grouping all accept the same number of valence electrons, simply they reside in successively higher shells as yous get down the group. This is what gives the elements in a grouping similar properties. For instance all the main group elements with four valence electrons are in Group 14 starting with carbon. They all have their valence electrons in s and p subshells. Those iv s and p electrons will comport similarly regardless of shell they are in.
In addition to dividing the tabular array into groups and periods the table can be divided into blocks (see Periodic table filled by blocks) where the last subshell in which the atom's outermost electrons reside determines the "block" to which it belongs. Carbon, for example, is in the p-block considering its last electrons are in the p subshell.
The total number of electron shells an atom has determines the period to which information technology belongs. Since each beat is divided into different subshells, as we step through the elements by atomic number, the subshells will fill with electrons roughly in the order shown in the table below (in the table the numbers refer to the crush and the letters to the subshell):
| Subshell: | S | G | F | D | P |
| Period | |||||
| 1 | 1s | ||||
| 2 | 2s | 2p | |||
| 3 | 3s | 3p | |||
| 4 | 4s | 3d | 4p | ||
| five | 5s | 4d | 5p | ||
| 6 | 6s | 4f | 5d | 6p | |
| 7 | 7s | 5f | 6d | 7p | |
| viii | 8s | 5g | 6f | 7d | 8p |
Hence the structure of the tabular array. Since the outermost electrons decide chemical properties, those with the same number of valence electrons are grouped together.
Encounter also
- Atom
- Atomic mass
- Atomic number
- Chemic element
- Electron configuration
- Isotope
- Metal
- Nonmetal
- Transition metal
References
ISBN links support NWE through referral fees
- Bouma, J. 1989. An Awarding-Oriented Periodic Table of the Elements. J. Chem. Ed. 66:741.
- Cotton, F. Albert, Yard. Wilkinson, C.A. Murillo, and M. Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed. New York: Wiley. ISBN 0471199575
- Greenwood, N. North., and A. Earnshaw. 1997. Chemistry of the Elements, second ed. Oxford: Butterworth-Heinemann. ISBN 0750633654
- Mazurs, Edward Yard. 1974. Graphic Representations of the Periodic System During Ane Hundred Years. University, AL: Academy of Alabama Press. ISBN 0817332006
- Scerri, Eric R. 2007. The Periodic Tabular array: Its Story and Its Significance. Oxford: Oxford Academy Press. ISBN 978-0195305739
External links
All links retrieved February 7, 2019.
- Periodic table. WebElements.
- Periodic Table. Large full-color scalable.
- Periodic table of Technology.
| Chemistry | |
|---|---|
| Belittling chemistry • Biochemistry • Bioinorganic chemical science • Bioorganic chemistry • Chemical biology • Chemistry education • Click chemistry • Cluster chemistry • Computational chemistry • Electrochemistry • Ecology chemical science • Green chemical science • Inorganic chemistry • Materials science • Medicinal chemistry • Nuclear chemistry • Organic chemistry • Organometallic chemical science • Pharmacy • Pharmacology • Physical chemical science • Photochemistry • Polymer chemical science • Solid-country chemical science • Supramolecular chemical science • Theoretical chemistry • Thermochemistry • Moisture chemistry | |
| List of biomolecules • List of inorganic compounds • List of organic compounds • Periodic table | |
| General subfields inside the Natural sciences |
|---|
| Astronomy | Biology | Chemistry | Earth science | Ecology | Physics |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Eatables CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due nether the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this commodity click here for a list of acceptable citing formats.The history of before contributions past wikipedians is accessible to researchers here:
- Periodic_table history
- Periodic_table_group history
The history of this article since information technology was imported to New World Encyclopedia:
- History of "Periodic tabular array"
Note: Some restrictions may apply to use of private images which are separately licensed.
Source: https://www.newworldencyclopedia.org/entry/Periodic_table
Post a Comment for "What Determines the Properties of Each Family on the Periodic Table"